
Building Cure and Seattle Children’s Therapeutics are devoted to developing innovative therapies for childhood disease. Meet the first patient to receive a cell therapy treatment produced at Building Cure.
When Building Cure opened in fall 2019, Meagan Hollingshead and Josh Chittim had more pressing concerns. Their normally energetic 6-month-old daughter Harper was sick, and multiple visits to their doctor in Yakima had provided no answers.
But when Harper’s condition worsened and she started struggling to breathe, they took her to the emergency room, where bloodwork revealed the devastating cause: Harper had acute lymphoblastic leukemia (ALL).
The doctor immediately sent them to Seattle Children’s.
“Meagan and Harper flew over to Seattle Children’s,” Chittim said. “And I drove there at 110 miles an hour.”
At that point, Hollingshead and Chittim weren’t aware Building Cure existed. They didn’t know how important the building, and the Seattle Children’s Therapeutics team it houses, would become to Harper’s future. And they had no idea Harper would receive the first cell therapy product manufactured there.
“She didn’t do anything they expected her to do”
Harper went straight to the Intensive Care Unit (ICU) at Seattle Children’s.
“They had a hard time getting IVs into her and intubating her,” Chittim said. “She actually crashed the first day because she was so sick.”
Harper spent the next eight months in the hospital as a multidisciplinary care team, led by Drs. Kasey Leger and Brittany Lee from the Cancer and Blood Disorders Center, treated her. When the initial chemotherapy had no effect, the team placed her on a study medication called azacitidine, hoping to “sensitize” her body to chemo.
It didn’t work. Harper’s ALL was resistant to traditional chemo. She needed a bone marrow transplant, but first she needed to get into remission. Leger and Lee placed her on an immunotherapy called blinatumomab. To everyone’s relief, Harper seemed to improve, and Hollingshead and Chittim saw new hope for their daughter.
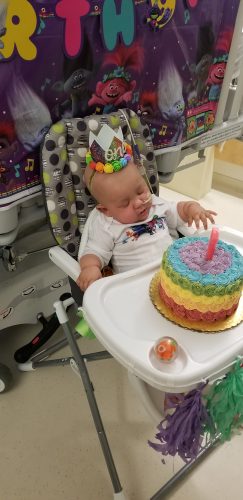
Just two weeks later, a bone marrow exam produced a startling result: Harper’s cancer had morphed from ALL to acute myeloid leukemia (AML) — a rare occurrence called lineage switch. This complication forced the team to put Harper on intensive myeloid-directed chemotherapy to treat the AML. It eradicated the AML, but unfortunately a small amount of her ALL persisted.
“It was a roller coaster,” Chittim said. “She didn’t do anything they expected her to do.”
The team once again used blinatumomab to effectively treat Harper’s ALL. Eventually, she was ready for a bone marrow transplant.
Harper received her transplant on April 3, 2020. Everyone was hopeful. But once again, things didn’t go as planned. In late April — mere days after Harper’s first birthday — tests revealed her ALL had returned.
While blinatumomab had worked twice, the team was hesitant to use it again because Harper’s bone marrow transplant was so recent. Instead, Leger and Lee filed a compassionate use request with the Food and Drug Administration and Seattle Children’s Institutional Review Board to give Harper a medication called venetoclax. Their request was approved, but the medication had no effect.
They decided to try blinatumomab again.
“Within a week it eliminated about 98% of her leukemia,” Hollingshead said. “That was a breakthrough for science because the team didn’t think it would work so close to her transplant. She was on it for six rounds: 28 days of infusion, a couple days off, then back on.”
With Harper’s ALL under control, Leger and Lee recommended chimeric antigen receptor (CAR) T-cell therapy, which they hoped would eliminate the remaining ALL and put her into long-term remission.
That’s where Building Cure and Seattle Children’s Therapeutics enter the story.
A building with a singular mission
Building Cure is devoted to research and developing innovative therapies for childhood disease. In addition to the Science Discovery Lab, Building Cure is the new home of the Therapeutic Cell Production Core (TCPC), also called The Cure Factory, where immunotherapy “products” (treatments for specific patients) are made.
The TCPC has almost three times as much square footage in Building Cure than in the previous location at Olive Lab. That extra space provides more than elbow room — it dramatically increases Seattle Children’s immunotherapy production capacity. That’s important because one of the primary limitations in immunotherapy research is manufacturing space.
The TCPC and Seattle Children’s entire cancer immunotherapy research program are part of Seattle Children’s Therapeutics (SCTx), which is nested within the Research Division. Seattle Children’s Therapeutics is a nonprofit enterprise devoted to envisioning and testing next-generation cell and gene therapies for pediatric diseases. It provides research and development, therapeutics manufacturing, FDA regulatory affairs compliance, clinical trials management and administrative functional support. Partners include the Cancer and Blood Disorders Center, the research centers at Seattle Children’s Research Institute, research’s Center Support Services, Seattle Children’s Foundation, and CureWorks member hospitals.
Immunotherapy is an exciting field and, bolstered by philanthropic support, Seattle Children’s is at the forefront.
“Immunotherapy is using the resources in your immune system to fight cancer the same way your body would fight an infection,” shared Dr. Rebecca Gardner, Seattle Children’s former Medical Director of Immunotherapy and Interim Chief Medical Officer of Seattle Children’s Therapeutics. “What makes it really appealing is that your immune system can have memory. When you get chemotherapy, once the chemo is gone, there’s nothing in your body to prevent the cancer from recurring. Whereas with immunotherapy, there’s the potential to create memory so that if your cancer came back, your immune system could recognize it, kick in and get rid of it.”
CAR T-cell therapy is a specific type of immunotherapy that involves extracting T cells from a patient’s blood (a process called apheresis), adding a new gene into the T cells that programs them to recognize a protein that’s expressed on the surface of a cancer cell via a chimeric antigen receptor (CAR) and then transfusing those modified T cells (now called CAR T cells) back into the patient. In essence, the CAR T cells are programmed to destroy specific types of cancer cells.
While the science behind immunotherapy is fascinating, its impact on the lives of kids like Harper will be its legacy.
A landmark “first”
Harper received a CAR T-cell infusion in early January 2021. Those cells were manufactured at Olive Lab, prior to the TCPC moving into Building Cure. Her infusion took on even greater significance when, just days before the infusion, tests detected leukemia cells in her spinal fluid, indicating that she urgently needed more treatment.
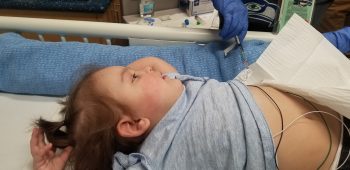
To everyone’s delight, Harper’s tests after the CAR T-cell therapy came back clear. The next challenge became keeping her in remission long term. To do that, Harper was enrolled in a clinical trial that “feeds” the CAR T cells with antigen-presenting cell (APC) boosters.
“CAR T cells are happy if they can find something with the target antigen to chew up and spit out,” Dr. Rebecca Gardner, Seattle Children’s former Medical Director of Immunotherapy and Interim Chief Medical Officer of Seattle Children’s Therapeutics said. “From the early studies we realized that if patients didn’t have enough antigen in their body when they got their CAR T cells, they were more likely to lose those CAR T cells early on. Dr. Colleen Annesley, lead for the leukemia program within Seattle Children’s Therapeutics, is running a trial where patients initially get their CAR T cells and then, after recovering from that initial infusion, they get a second product we manufacture called T APCs. We take their T cells and, rather than making them CAR T cells, we have the cells express a truncated version of the CD19 protein, which gives the CAR T cells something to feed on and hopefully keeps them around longer.”
In February, Harper’s T-APC boosters were the first immunotherapy products manufactured at Building Cure. She has received her first two boosters and, if all goes according to plan, will receive four more at a rate of one per month.

Harper’s tests continue to be clear, and she’s blossoming into a happy, boisterous child. She even got to return home to Yakima briefly, with the potential of returning home for longer periods between future boosters. While Harper’s journey is far from over, her family and care team have hope, and they want other families in similar circumstances to have hope too.
“It’s hard to see your child go through all that pain,” Hollingshead said. “But I want parents to know not to give up on their kids. Just keep fighting for them.”
Building Cure and Seattle Children’s Therapeutics are key allies in that fight. And as the field of immunotherapy grows and community support accelerates research discoveries, Seattle Children’s Therapeutics team members hope to someday use their innovations to treat other diseases in addition to cancer.
It’s all about living up to that name: Building Cure.
As part of the It Starts With Yes Campaign, donations from our generous community have been a catalyst in accelerating pediatric cancer immunotherapy clinical trials, opening Building Cure, collaborating with leading scientific partners, and launching Seattle Children’s Therapeutics.

