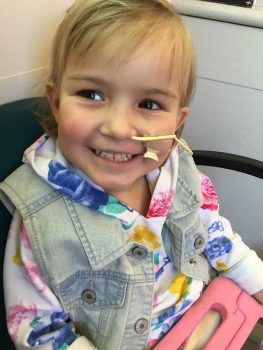
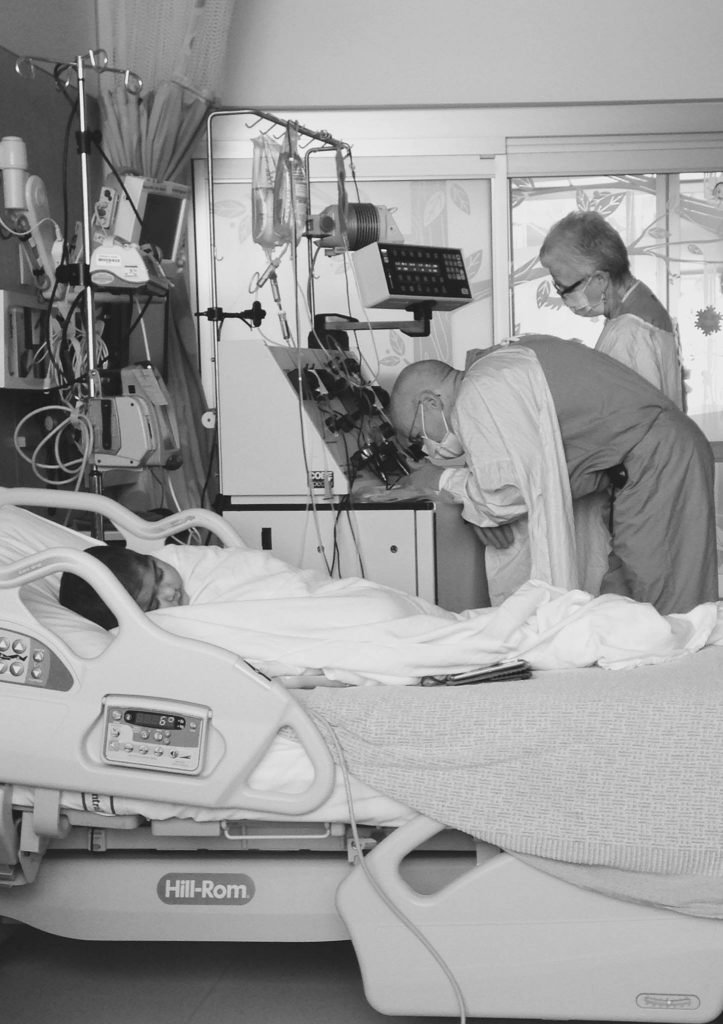
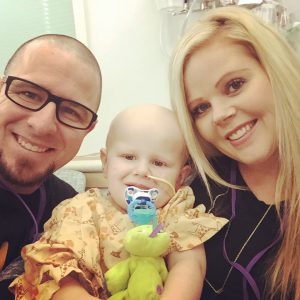
When Emmy Cole was 2 years old, her mother noticed her struggling to walk. She grabbed her cell phone and tearfully recorded Emmy wince in pain as she took only a few, small steps. She knew something was terribly wrong with her daughter. Immediately after, they came to Seattle Children’s in search of answers. Emmy […]
EDITOR’S UPDATE: The U.S. Food and Drug Administration approved the cancer drug Vitrakvi (previously known as larotrectinib). Vitrakvi is indicated for the treatment of adult and pediatric patients with metastatic or unresectable solid tumors that have a NTRK gene fusion without a known acquired resistance mutation, and have no satisfactory alternative treatments options or whose cancer has […]
With 2018 in full effect, On the Pulse is taking a moment to hit rewind to share five stories that might have floated beneath the flurry of headlines in 2017. We invite you to take a look back at some of last year’s stories that inspired us and gave us hope. 1. A Mother’s Intuition […]
Today, rounds of a different kind were made. Instead of doctors in white coats, the Seattle Seahawks and members of the Sea Gals, dressed in blue and green, made their way through the hospital to visit patients and families at Seattle Children’s. They couldn’t have picked a better day to bring cheer to 12s in […]
This Halloween marked a monumental milestone for 7-year-old Erin Cross. For the first time in Erin’s life, she was healthy enough to go trick-or-treating. And her costume of choice – an old woman – held a special meaning for her family. Two years ago, Erin’s family was facing the devastating reality that they may never […]
Seattle Children’s is getting set to launch a program that will redefine how we care for children with “high-risk” leukemia – or leukemia that doesn’t respond well to standard treatments and/or has relapsed after therapy. Unfortunately, less than 40% of children with high-risk leukemia will live for more than four years after they’re diagnosed. Our […]
To pass the nearly 180 days she was a patient in Seattle Children’s Cancer Unit with graft-versus-host disease (GVHD), London Bowater took orders from her doctors, nurses and other patients and families for friendship bracelets that she would braid from her hospital bed. While her handicraft would help fill the time between treatments, it did […]
September is Childhood Cancer Awareness Month. But What does ‘awareness’ really mean? To become aware? To obtain new knowledge? To gain a new perspective? To become informed? To become concerned or even empathetic to an unfamiliar situation? The concept of awareness can take on many faces, and its perception can change depending on the person […]
In just three days, 9-year-old Wyatt Zender and his family will see his artwork come to life on the Chicagoland Speedway. Wyatt, a cancer patient at Seattle Children’s, was the lucky winner of a coloring contest presented by Great Clips to design the paint scheme for Kasey Kahne’s No. 5 Great Clips Strong Against Cancer […]
Liesel Von Imhof, 18, came to Seattle Children’s from her home in Anchorage after learning the reason for her migraines: a ping-pong ball–sized tumor in the middle of her brain. In honor of Childhood Cancer Awareness Month, here she shares her journey of diligently working to achieve her goals despite recovering from brain tumor surgery […]