
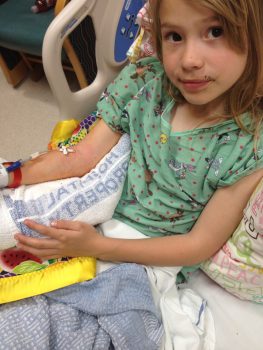
When 17-year-old Ellie Osterloh spins high above the ground from a lyra, a circular hoop used in aerial acrobatics like Cirque du Soleil, she feels empowered.
“On the lyra, it’s an incredible feeling to be so high in the air with no harnessing,” Ellie said. “It’s a lot of adrenaline and I feel like I can do anything.”
Now, thanks to Trikafta, a new drug approved in October 2019 by the U.S. Food and Drug Administration (FDA), Ellie, who participated in the clinical trial for the drug, shares a similar zeal for her future.
“Even though it’s hard sometimes to be so optimistic, I’ve always thought it might be possible to go to college and have kids and a family of my own,” she said. “It’s crazy how my outlook has changed. I’m still processing all the possibilities.”
That’s because Ellie has lived her entire life with cystic fibrosis, a rare, progressive, life-threatening disease. She had her first appointment with Dr. Bonnie Ramsey, the director of Seattle Children’s Center for Clinical and Translation Research, still in her mother’s womb. Hours after birth she was transferred to Seattle Children’s where she began intensive therapy that she’s continued over the last 16 years.
“This is a really big step forward for Ellie and other people living with cystic fibrosis,” said Ramsey, a pioneer in cystic fibrosis treatment. “Ellie is a highly talented young lady with a bright future ahead of her.”
Trikafta offers robust third-generation therapy
According to the FDA, cystic fibrosis results in the formation of thick mucus that builds up in the lungs, digestive tract and other parts of the body. It leads to severe respiratory and digestive problems as well as other complications such as infections and diabetes. Cystic fibrosis is caused by a defective protein that results from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The most common of these mutations is the F508del mutation.
Trikafta is the first triple combination therapy available to treat patients 12 years and older with cystic fibrosis who have at least one F508del mutation. Other previously approved therapies for cystic fibrosis treated patients with two copies of the F508del mutation. Trikafta has the potential to treat up to 90% of all people with cystic fibrosis.
“The first therapy approved for cystic fibrosis treated only about 7% of patients,” said Dr. Ron Gibson, director of Seattle Children’s Cystic Fibrosis Program. “Not only is this third-generation drug more robust, but it is treating the vast majority of patients with cystic fibrosis.”
Breathing clearer
Trikafta works by correcting the malfunctioning CFTR gene to restore normal activity. It is made up of three different drugs – elexacaftor, tezacaftor and ivacaftor – that target the defective CFTR protein.
As part of the phase 3 clinical trial, Ellie received a study drug with a very similar profile to the approved therapy. In addition to her regular therapies, which includes daily chest percussion therapy to clear mucus from her lungs and enzyme replacement therapy, she took two pills in the morning followed by another pill at night.
Ellie noticed a difference within weeks after taking the triple combination therapy.
“I was coughing less and breathing clearer,” she said. “I had more of an appetite so I was eating more.”
Her respiratory function tests and sweat chloride levels improved too. Since she started taking the drug about a year ago, she’s grown nearly seven inches.
“Before starting the trial, I was at a really low point. I was getting pretty sick,” Ellie said. “It means so much to have this drug as an option, not just because it’s helped me, but also because I have people close to me who need this drug too.”
Results gathered from over 500 patients in two multi-center phase 3 trials painted a similar picture. In an editorial published in the New England Journal of Medicine, the National Institutes of Health director, Dr. Francis Collins said the results “document impressive benefits from triple-drug therapy” and “should be a cause for major celebration.”
A mother’s legacy

Growing up, Ellie’s mom, Laura Osterloh, kept a close eye on her daughter’s therapies and care. She helped Ellie stay on top of her two to three hours of daily therapy. She and Ellie would often make extra trips from their home near Bellingham to Seattle, so that Ellie could see her Seattle Children’s care team.
When Laura was diagnosed with a terminal illness, one of her wishes was that Ellie would have the opportunity to participate in a clinical drug trial as soon as one became available for her type of cystic fibrosis. One month after Laura passed away in December 2015, Ellie enrolled in one of the early clinical trials sponsored by Vertex, the pharmaceutical company behind Trikafta.
“That was the beginning of Ellie being in the Vertex study,” said Sharon McNamara, a research nurse manager overseeing the cystic fibrosis clinical trials at Seattle Children’s.
Over the years, McNamara has formed a close bond with the Osterloh family. She recalls the appointment when Ellie and her family received the first functional test results after starting the triple-drug therapy – the response was dramatic.
“I was shocked when I saw how much my numbers had improved after just one month,” Ellie said. “Dr. Bonnie, my dad and my stepmom were all crying. It was very emotional because we knew how much this would have meant to my mom. All she ever wanted was for these drugs to be approved.”
Don Osterloh, Ellie’s father, echoed this sentiment.
“Laura would have been so excited to see this obviously for Ellie, but also for those with cystic fibrosis in our extended family and for all the others who now have access to this drug,” he said. “It’s life-changing for all of them.”
Leader in cystic fibrosis research

Working alongside colleagues at the University of Washington School of Medicine, Ramsey, Gibson, McNamara and their team at Seattle Children’s have made significant contributions to advances in cystic fibrosis treatment over the last thirty years. Their work has led to improvement in the long-term prognosis and quality of life for children with cystic fibrosis.
Patients like Ellie who participate in early and late phase clinical trials are critical to this success.
“It’s important to participate in research because you never know what might be possible,” Ellie said.
Ellie is continuing to take part in research related to Trikafta. Seattle Children’s has also opened a study of Trikafta in patients age 6-11.
“We do have to remember the somewhere between 7-10% of those living with cystic fibrosis still have no targeted treatment,” Ramsey said. “We feel strongly that our work is not done until those patients have a better treatment option as well.”
For more information on cystic fibrosis research trials at Seattle Children’s, please call 206- 987-3921 or visit our current research studies page.
Resources
- Seattle Children’s Cystic Fibrosis Program
- Landmark studies in the New England Journal of Medicine and the Lancet

