As Director of Cardiac Catherization Labs at Seattle Children’s, Dr. Brian Morray routinely performs diagnostic as well as complex heart procedures on patients as young as a few days old all the way up to adults. Part of his job is to provide minimally invasive services for children with congenital heart disease (CHD).
“Congenital heart disease is the most common kind of congenital birth defect,” said Morray. “For the vast majority of our patients, it occurs when the heart is developing in utero. It’s a structural abnormality of the heart that we frequently diagnose before birth.”

An orphan disease
Every year in the United States, more than 40,000 children are born with CHD. Depending on the severity and type of defect, a child may require an intervention to survive within the first few weeks of life.
Forty thousand children may sound like a lot but, in terms of the general population, it’s a relatively small number compared to common adult diseases. “CHD is considered an orphan disease,” said Morray. “This makes it difficult to develop products specifically for children with heart disease.”
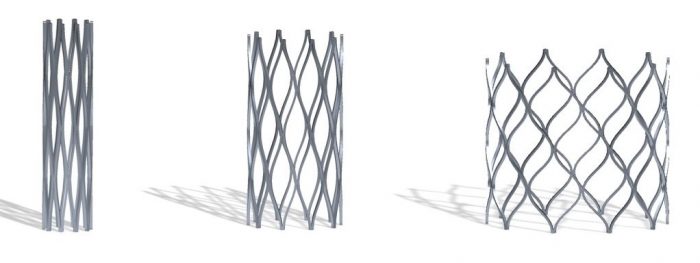
Products like heart stents that fit a newborn with CHD.
“Typically, it’s been our practice for many decades to adopt technologies that have been developed for adults and apply them to children,” said Morray. “The term is ‘off-label.’ But there’s a problem with the size of adult stents. Particularly for small children, the catheters or stents that are used can be too large. We know how to make it work, but we’re using applied technology that’s not specifically designed for kids.”
“There are very few technologies that are developed specifically for children,” said Morray. “Very few. I can count on probably one or two hands the number of devices that have specifically been tested and designed for children with congenital heart disease.”
Growth is another issue. “Stents are not designed to grow with the patient,” said Morray. “They’re designed to be put into adults who are done growing, which is obviously not the case with children, particularly a small child.”
This means children with CHD often receive small stents that are not designed to expand to the size of large adult blood vessels.
But a new engineering company with a focus on device development for children may change all that. Morray and Seattle Children’s are at the forefront of making it happen.
A stent that grows with the child
The Renata Minima Stent is the first product of Renata Medical, a young engineering company focused on designing and developing products for the unmet needs of pediatric patients. The Minima stent is designed to be implanted in small diameter blood vessels in infants and small children and can be gradually expanded up to adult size over the course of the child’s lifetime through a series of minimally invasive procedures.
“Developing a stent that’s small enough for an infant but can accommodate for growth is an engineering challenge that most companies have not been willing to take on,” said Morray.
“The fact that this stent is designed specifically for small children and infants is very unique. It is rare that a company would have any interest in developing something specifically for this small and vulnerable population.”
Early feasibility trial
The company tapped Seattle Children’s to help with the early use of this technology through what’s called an early feasibility study.
“This is a relatively new pathway that the Food and Drug Administration has created to bring products to market early in their development,” said Morray. “It allows companies to continue to iterate on their designs during clinical use so that they can be made better through clinical experience. This allows companies and investigators in the United States to become early adopters of these new technologies and bring them to our patients.”
Seattle Children’s will be one of only four pediatric institutions to participate in the early feasibility study, officially known as the Multicenter Early Feasibility Trial of Neonatal, Infant, and Young Child Vascular Stenoses Studying the Renata Minima Stent. The trial will involve a total of ten participants with native or recurrent narrowing of large blood vessels called the aorta or pulmonary artery.
Seattle Children’s will run its portion of the study through the Center for Clinical and Translational Research (CCTR), part of Seattle Children’s Research Institute. CCTR provides the infrastructure and knowledge Seattle Children’s researchers need to develop innovations that will advance pediatric care and speed improvements to children’s health.
Morray credits his predecessor and mentor, Dr. Tom Jones, and CCTR research coordinator Elizabeth McKinney, for building the reputation of Seattle Children’s as a place where thoughtful, thorough, accurate and timely clinical cardiac investigations occur.
“These types of studies require an incredible amount of accuracy, diligence and attention to detail,” said Morray.“The data have to be accurate and submitted in a timely fashion. Companies looking for clinicians and investigators to help them study their products want investigators who are not only good interventional cardiologists, but have a lot of experience navigating regulatory waters.”
Morray says that because of the work done by Jones and McKinney, Seattle Children’s has been involved in the majority of cardiac device trials in children over the last 15 to 20 years. He looks forward to carrying on his predecessor’s work. “Dr. Jones made sure Seattle Children’s was involved in much of the growth of this field in the last 30 years. Now it’s our responsibility to continue that as best we can.”
Being involved with the Renata Minima Stent is a good start.
Seattle Children’s Heart Center has one of the largest and most accomplished teams of cardiac experts in the country with more than 50 board-certified cardiologists and cardiac surgeons. Our teams of specialists perform more complex pediatric heart procedures than any other provider in the Washington, Wyoming, Alaska, Montana and Idaho region.

