
Doctors at Seattle Children’s are investigating whether a simple liquid biopsy containing a small amount of fluid from a patient may someday provide an easier route to a genetic diagnosis in children with vascular or lymphatic malformations.
The work is a collaborative effort led by Dr. James Bennett, a clinical geneticist and co-director of the molecular diagnostic laboratory at Seattle Children’s and Dr. Jonathan Perkins, an otolaryngologist and director of the Vascular Anomalies Clinic. Liquid biopsy offers an alternative to the more invasive surgical biopsies required – when a genetic, or molecular diagnosis, is needed to help guide a patient’s treatment.
“We can now provide a specific genetic diagnosis for a lot of vascular malformations,” Bennett said. “That’s important for families for a variety of reasons with one being it’s just extremely healing and powerful to know the reason why your child has these differences.”
Bennett adds that many of the genetic causes behind vascular malformations are in the same pathways involved in different adult cancers. Several drugs approved for cancer target these pathways by calming down overactive cell growth. A number of these drugs are already being tested in clinical trials for children with vascular malformations, but a genetic diagnosis is needed to determine if a child is a candidate for these studies.
“A liquid biopsy offers a fast pass to get a specific molecular diagnosis without necessarily having to do a surgery on a child,” Bennett said. “If we can convert our research into a clinical grade test, we have the opportunity to potentially make a lot more kids eligible for the clinical trials investigating new drugs to treat vascular malformations.”
How liquid biopsy works

Lymphatic malformations occur in about 1 in every 4,000 births when the tubes that carry lymph fluid throughout the body form abnormally. Most often, lymphatic malformations are in the head and neck, sometimes forming large fluid filled cysts. The vessels inside the cysts may shed their DNA into the surrounding fluid.
Bennett was interested in identifying alternative ways to make a genetic diagnosis in patients with vascular malformations without the need for surgery, and Perkins hypothesized that a little fluid taken directly from the cyst could provide enough DNA from the malformation to hunt for any genetic mutations.
“When you take a fluid sample from a patient and you spin all the cells in a centrifuge, the cells go to the bottom and you get this layer of liquid on top,” Bennett said. “It turns out there’s tiny little molecules of DNA, called cell-free DNA, that float around in that liquid. It’s called cell-free because the DNA is not inside a cell. We can then analyze this cell-free DNA for mutations present in the patient’s malformation using genetic tests.”
A paper published in Genetics in Medicine by Bennett, Perkins and others from Seattle Children’s Center for Clinical and Translational Research and Center for Developmental Biology and Regenerative Medicine used the liquid biopsy approach to identify genetic mutations in the cyst fluid cell-free DNA in patients with lymphatic malformations.
First, they tested the approach using a bank of vascular malformation patient tissue samples stored at Seattle Children’s in which the genetic diagnosis was already known. Liquid biopsy of cyst fluid cell-free DNA identified the previously known mutations in all seven lymphatic malformation samples. Prospective testing of cyst fluid cell-free DNA in lymphatic malformation patients who had never undergone surgery identified a genetic cause in four out of five of those enrolled in the study. The liquid biopsy did not find genetic mutations in the plasma from patients with lymphatic malformations but did find mutations in plasma from blood samples taken from patients with other types of vascular malformations.
“This gives us the first proof of principle that we can detect genetic mutations using a liquid biopsy of cyst fluid from children with lymphatic malformations,” Bennett said. “This is significant because the procedure to draw the fluid from the cyst is much less invasive and complicated than surgically removing the tissue needed in the operating room.”
Why liquid biopsy was right for one family
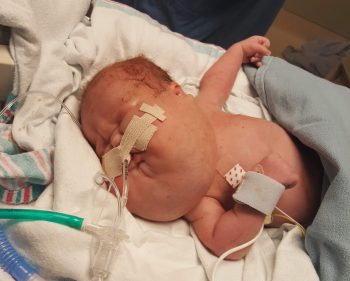
Today, Ezra Anpo, 2, zooms around the room chasing his older sister. The fact he is living a normal childhood means everything to his parents, Chelsea Gillis and Hideki Anpo.
When Gillis was 20 weeks pregnant with Ezra, an ultrasound showed a concerning lymphatic malformation near his neck. The malformation continued to grow and at 28 weeks pregnant, his parents received a referral to see Perkins.
“Ezra has a lymphatic malformation that’s more extensive than most,” said Perkins. “It’s location near the back of his tongue means that any significant inflammation could block his airway and send him to the hospital or into surgery.”
On January 9, 2019, Ezra was born at the University of Washington Medical Center and was transferred immediately to Seattle Children’s Neonatal Intensive Care Unit (NICU). The team in the NICU stabilized Ezra’s breathing and supported his feeding while Seattle Children’s vascular anomalies care team determined a longer-term treatment plan.
Given the growing number of options available, Perkins wanted Ezra to undergo genetic testing to confirm his diagnosis and help guide his treatment. However, the standard approach of taking a biopsy from the malformation during a surgery also put Ezra at high risk of damaging his airway. If the airway became compromised, he would need a tracheostomy to breathe.
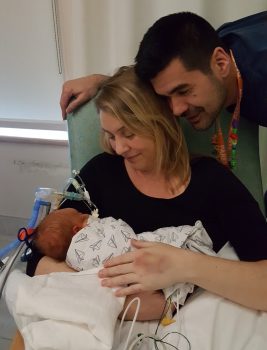
“There was a lot of concern about doing any type of surgery that could cause an inflammatory reaction, which would most likely result in a tracheotomy,” Gillis said. “Both dad and I were adamant that we wanted to do everything possible to avoid a tracheostomy.”
Perkins told the family about the liquid biopsy approach in development. Since he would only need to draw fluid from the cyst using a needle, it offered a significantly less invasive, and less complicated way to confirm Ezra’s genetic diagnosis.
The family agreed to move forward with the experimental test. Perkins took the fluid from Ezra’s cyst during a procedure to place a gastrostomy tube.
Shortly after, the family and Perkins had answers from the liquid biopsy: Ezra had a mutation in a gene called PIK3CA. Starting treatment with an immunosuppressant drug could provide initial benefit until he was old enough to potentially enroll in a clinical trial of a therapy that targets his mutation.
Toward a clinically available option
For now, the results of Ezra’s test are for research use only, though Bennett is hoping to develop a clinically available option in the near future.
Earlier this year, Bennett received a $2.5 million, five-year Research Project Grant (R01) grant from the National Institutes of Health to continue studying the use of cell-free DNA in larger numbers of patients with vascular malformations. Another area Bennett is excited to explore is whether the liquid biopsy could serve as a biomarker for patients receiving medication therapy.
“Once a child is on a drug targeted to a specific mutation and you’re trying to figure out if the drug is helping or not, you could potentially use this test to look for trends in the mutated DNA over time,” he said. “If levels of this DNA are trending down, it could indicate a drug is working.”
Leader in vascular anomalies care and research
A precision diagnostic test developed by Seattle Children’s is already widely used in patient care. It is the only genetic testing panel certified for clinical use with vascular malformations at a children’s hospital.
The Vascular Anomalies Sequencing Panel, or VANseq, tests for mutations in 44 genes known to cause vascular anomalies. Doctors can use results from VANseq to make treatment decisions or help qualify patients for clinical trials. The advanced test currently accepts blood, saliva or tissue specimens obtained from the patient, but Bennett believes their research is on path to add testing with cell-free DNA to the clinical test.
“There are labs doing clinical grade testing with cell-free DNA, mostly for cancer,” Bennett said. “We have more work to do to before the cell-free test for vascular anomalies is ready for clinical use, but I’m confident we’ll get there.”
Beyond diagnostics, Seattle Children’s has long led advances to improve care for children with vascular anomalies. A collaborative team of physician-scientists work together to bring innovations such as glue embolization and facial mapping to the nearly 2,000 children the program sees each year.
With two clinical trials investigating the use of targeted therapies for vascular malformations already open at Seattle Children’s, Perkins sees an opportunity to move the field forward yet again.
“It’s completely shifting the paradigm of treatment of vascular malformations from surgery to medical therapy,” Perkins said. “We can get to the root of what’s causing the malformation and using that information, develop new treatments that hopefully will be significantly more effective than what we’ve had to offer before.”
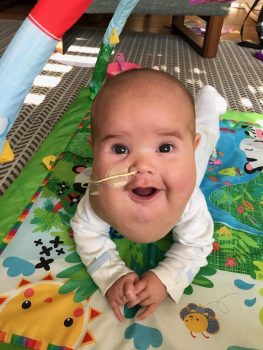
“Ezra is thriving”

The collaborative model embraced by Perkins, Bennett and the vascular anomalies team has helped Ezra’s parents feel supported through their son’s hospitalization and ongoing care.
“I’m thankful because we took a good path with Ezra,” Anpo said. “The part I appreciate most is that it was a team effort. We were involved in the decision making and I felt like we got the information we needed from his whole medical team to guide us to the right decision.”
Perkins estimates if Ezra had needed a tracheostomy, he would have had a much longer hospital stay upfront and then likely needed several trips to the operating room in his first two years of life. His mother is grateful they were given the option to avoid surgery and get a genetic diagnosis through the research study.
“We are very appreciative that Dr. Perkins listened to us and really took our overall goals into account when making decisions about our son’s care,” she said. “We’re so fortunate. We haven’t needed any major surgeries, and Ezra is thriving. He’s fully oral besides his meds, his breathing is great and he’s developing normally. He’s so full of life.”

