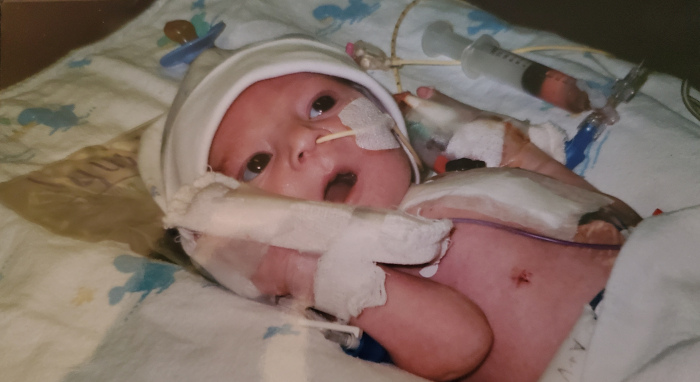
 Worry flooded Candice Andrews’ mind as doctors wheeled her newborn son away for open heart surgery.
Worry flooded Candice Andrews’ mind as doctors wheeled her newborn son away for open heart surgery.
“I knew about his heart condition since I was 7 months pregnant,” Andrews said. “However, it was still very scary knowing that someone was going to do surgery on my 7-day old baby.”
Andrews’ son, Marcus, was born with hypoplastic left heart syndrome, a rare and serious birth defect that occurs when the left side of the heart is not fully developed.
Fortunately, Marcus recovered well after his first of what would be three surgeries needed to treat his heart condition.
“Doctors mentioned how exceptional his recoveries were,” Andrews said. “We were so grateful, given how unknown the entire situation was for us.”
Although his first few years of a life were a bit rocky, Marcus remained relatively healthy as he progressed through childhood.
Helping others through research
 In 2017, when Marcus was 14 years old, Dr. Mark Lewin, division chief of cardiology and co-director of Seattle Children’s Heart Center, told his family about a clinical trial that Marcus would be a good candidate for.
In 2017, when Marcus was 14 years old, Dr. Mark Lewin, division chief of cardiology and co-director of Seattle Children’s Heart Center, told his family about a clinical trial that Marcus would be a good candidate for.
“Although Marcus has lived a healthy life, we were still very much open to participating in the trial,” Andrews said. “We’ve always felt that if there’s anything we can do for research in developing new protocols or new ways to help other families dealing with this heart condition, we wanted to be part of those efforts.”
The study, called Fontan Udenafil Exercise Longitudinal Assessment Trial (FUEL), evaluated the clinical efficacy and safety of udenafil, an new oral medication for the treatment of adolescent subjects who have undergone the Fontan procedure. The Fontan procedure is the final of three surgical procedures that children like Marcus undergo to help rebuild parts of the heart and redirect the way the blood flows.
Agreeing to participate in the study, Lewin introduced the family to Dr. Matthew Files, who led the clinical trial at Seattle Children’s.
“During the time Marcus was born, we focused primarily on survival for this group of patients,” Files said. “While challenges remain, the survival for these children has steadily improved. Now, most of those who undergo multiple surgeries to manage single ventricle heart defects as children continue to experience a good quality of life through their teenage years; however, when they enter early adulthood we found many become sick and potentially may need a heart transplant because circulation in the heart becomes less efficient and less functional. That is why we’re now looking for ways to maximize health, quality of life, and longevity in this specific population.”
With over 400 patients enrolled, the FUEL study is the largest multisite study to be completed in the world of congenital heart disease. Seattle Children’s was the only hospital in the region to offer the trial.
“We currently do not have any other therapies that prolongs efficient circulation in the heart or improves quality of life,” Files said. “This is the first medication we can lean on to try and improve a child’s long-term health status.”
Finding the fuel

Participating in the study was a new and exciting experience for Marcus.
“All I had to do to was add a medication to my daily routine,” Marcus said, “then wait to see what effect it had on my physical performance.”
At the beginning of the study, all patients ages 12-18 were given a questionnaire about their physical activity and quality of life. They then underwent blood work, genetic testing, echocardiograms, and EKGs to assess their heart structure and function.
“The main outcome measure was the result of the cardiopulmonary exercise test, a measure which looks at how well the heart and lungs and rest of the muscles work together,” Files said. “This is the single best way to assess someone’s overall cardiovascular health.”
At each trial site, half of the patients were given a placebo and half were given the drug for 6 months. At the end of the 6 months, patients repeated the tests, including the exercise test.
“After taking the medication as part of the study, there was an improvement in Marcus’s physical performance,” Files said.
Marcus could feel the difference.
“I noticed I could go much longer on the stationary bike during the exercise test,” he said. “I just felt better overall.”
Hopeful hearts
The results of the trial, published in the journal Circulation, reflected Marcus’s improvement in exercise performance.
“We took what we consider standard patients with Fontan circulation, ones doing relatively well, to see if the medication could sustain or improve overall cardiac health,” Files said. “Based on our trial results, we did see modest improvements in exercise performance in the treatment group, over the placebo group. We’re excited as this could be a treatment we could eventually recommend for all patients with this condition.”
While the team will continue to gather data in an extended phase of the clinical trial, Files is optimistic that this could change the medical field in the treatment of congenital heart defects.
“We’re in the process of applying to the FDA to get the medication approved to treat pediatric patients,” he said. “This is important because most medications used in pediatric cardiology are not as thoroughly studied in children and are based on adult studies.”
The family was happy that Marcus was able to participate in the study and help move the needle forward in finding innovative treatments for his condition.
“We’ve always got exceptional care at Seattle Children’s,” Andrews said. “They always want to know what we think and how we feel, and that’s so important when it comes to finding the best care for your child.”
For more information on research trials at Seattle Children’s, please visit our current research studies page.

