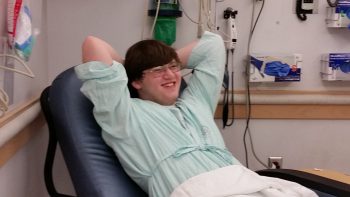
For the first time in his life, Shanahan “Shanny” Dameral, 19, has a girlfriend. Soon, he’ll be graduating with a high school diploma and looking for his first job on the Kitsap Peninsula.
What seems routine for many is a big deal for Shanahan and other children living with treatment-resistant or intractable epilepsy. For reasons largely unknown, seizures in this subset of children persist long past their discovery in early childhood despite being treated with multiple medications and undergoing surgery to remove the affected parts of their brain.
Diagnosed with epilepsy at age 5, life for Shanahan has always come with seizures attached. When his seizures returned after a second brain surgery shortly after his 16th birthday, his mom Linley Allen, hoped for a medical breakthrough.
“We needed to find something else since another surgery was out of the question,” Allen said. “We had heard about a drug being studied for a more severe seizure condition. I kept holding onto hope that it might be expanded to treat Shanny’s type of seizures because it was all we had at the time.”
Then last year, Shanahan’s longtime neurologist at Seattle Children’s, Dr. Russell Saneto, told the family about a phase 1 trial of an experimental therapy known as Nab-rapamycin (ABI-009) for patients with intractable epilepsy at Seattle Children’s.
“Dr. Saneto has always pushed for better ways to treat Shanny’s seizures, and even after he explained that this trial was early in the research process, Shanny popped right up and asked, ‘When do we start?’” said Allen. “I was like, ‘Shanny, this is going to be a couple of years out,’ so we were both surprised when Dr. Saneto said, ‘No, you can start next month.’”
Life with seizures attached
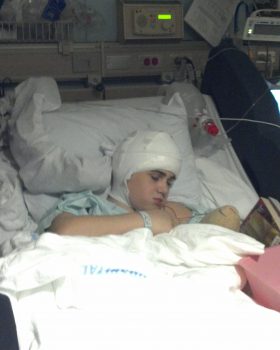
According to Seattle Children’s neurosurgeon, Dr. Jason Hauptman, uncontrollable or refractory seizures continue in about half of the children who undergo epilepsy surgery. When surgery fails to cure a child’s epilepsy, doctors and families do their best to manage the relentless seizures with existing antiepileptic drugs because few other options exist.
It required a joint effort from Hauptman, Saneto and their colleagues in Seattle Children’s Neurosciences Center and the Center for Clinical and Translational Research to open the Nab-rapamycin for Surgically-Refractory Epilepsy (RaSuRE) trial to investigate a new, potentially more targeted therapy for children with intractable epilepsy who continue to have more than eight seizures a month following surgery.
Few other research efforts in the U.S. are focused on developing new therapies for this subset of children even though, as Hauptman notes, identifying an effective therapy is a matter of life and death for many children after surgery fails to stop their seizures.
“It’s a question of what do you do for those children who have had the penultimate treatment, which is surgery with the intent to cure their epilepsy, and they still have seizures,” Hauptman said. “Life for these children is not only difficult, but the condition becomes more deadly with each year their seizures continue unchecked.”
Targeting mTOR in epilepsy

Developed by Aadi Bioscience, Nab-rapamycin is designed to target mTOR, an overactive protein implicated in a number of childhood brain disorders.
“This protein is present in virtually every cell in the body and is essential for regulating cell growth and function,” Hauptman said. “Because it also has important functions in brain cells, mTOR abnormalities can be a common denominator in a number of the epilepsies we treat.”
Given in conjunction with existing antiepileptic drugs, the hope is that Nab-rapamycin will decrease seizure severity by reducing the activity of mTOR. Another mTOR inhibitor similar to Nab-rapamycin has been used in the clinic to safely treat a seizure disorder called tuberous sclerosis complex (TSC). This drug improves seizures in children with TSC by targeting a known genetic mutation, which causes hyperactivity in the mTOR pathway.
“One goal of this trial is to expand our vast knowledge of mTOR beyond the lab and to start thinking of new ways to use a relatively safe medication to improve seizure control in a wider range of difficult to treat epilepsies,” he said.
Research moves from the bench to the bedside

Generous philanthropic support to generate the required pre-clinical data coupled with related research discoveries identified at Seattle Children’s helped propel the RaSuRE clinical trial into existence.
Dr. Ghayda Mirzaa, a researcher at Seattle Children’s Research Institute, studies the genetic causes of neurodevelopmental disorders. Mirzaa and her colleagues in the Center for Integrative Brain Research traced a group of brain disorders in children to mutations in a gene that produces the mTOR protein.
“Through our work we’ve been able to find genetic changes present in the brain tissue of children with intractable epilepsy,” said Mirzaa. “Most of these genetic changes cause activation of the mTOR pathway. Therefore, using inhibitors of the pathway is a very valid therapeutic approach, and it’s exciting to see the team bring a new potential therapy to life in a clinical trial for children with intractable epilepsy.”
In the future, Mirzaa expects knowledge to expand rapidly and that innovative models now in development will help clinical researchers better personalize treatment and even select the best drug candidates for clinical trials. By testing mTOR-targeting drugs first in culture, her lab and others can help clinicians identify which drugs might have the biggest impact for the greatest number of children, as well as predict the effect the drug will have when used in combination with other therapies. This precision medicine approach led by Mirzaa will enable Saneto and other clinicians to match children with therapies that target the Achilles heel of their hard-to-treat seizures.
“Since there is a lot of genetic variation from one child to another, it’s likely that a therapy that works for one child may not be effective in another child even though they both have mTOR mutations,” said Mirzaa. “Having the ability to model genetic variation in neuronal cultures in the lab will help identify the best treatment plan for children, which would be a major step forward.”
‘I would 100% want him to stay on this medication’
As part of the RaSuRE trial, Shanahan received three infusions of Nab-rapamycin at Seattle Children’s one week a part. He continued to take his five other medications as prescribed. Shanahan and his mom tracked any seizures he had over the five-month study period.
While the primary aim of RaSuRE is to assess the safety and tolerability of Nab-rapamycin with later trials being needed to determine whether it improves seizure control, Allen noticed a difference in her son’s seizures on the study medication.
“I would 100% want him to stay on this medication,” Allen said. “The biggest thing I saw is it shortened his seizures with no noticeable after effects.”
Including Shanahan, up to 18 children and young adults between the ages of 3-26 will receive Nab-rapamycin as part of the first phase of this research. Researchers anticipate that results from this trial will inform a larger, multi-center study, reaching children beyond Seattle Children’s in the next two to three years.
Holding onto hope

Shanahan and Allen are already looking ahead to the next opportunity to participate in additional studies of Nab-rapamycin. By sharing their story, Allen hopes she can inspire others to get involved, helping provide the critical funding that is needed to accelerate future clinical trials and the development of precision medicine approaches aimed at treating the root causes of a child’s epilepsy.
“If we don’t do these trials, we’re not going to find what’s going to help our kids with epilepsy,” she said. “We need to find better treatments so that our kids can be active, so that they can be a part of life.”
Until then, she has a new hope to hold onto – that a therapy specifically for her son’s condition is within reach. And with that comes hope for her son’s future too.
“When he graduates from his high school program next year, he’s able to work, he wants to work, but I’m worried whether someone will hire him because of his epilepsy,” she said. “He’s an incredible young man that’s just trying to make it in the world like we all are, and my hope is that these clinical trials will help make that a reality.”
For kids with complex neurological disorders who have few or no other treatment options, advances made by translating research results into therapies available at the bedside can offer new hope, and even a cure, which is one of the reasons we launched It Starts With Yes: The Campaign for Seattle Children’s. It Starts With Yes is a $1 billion initiative with a bold vision to transform children’s health. With your help, we will continue to provide financial assistance for families in need; expand necessary healthcare and research facilities; and invest in clinical and research programs to advance pediatric medicine. Give today to help support kids like Shanahan and transform childhood health for generations to come.
To learn more about participating in the RaSuRE trial, please contact Renée Rivers at 206-987-1697 or [email protected].
Resources
- More information on ClinicalTrials.gov
- Seattle Children’s Epilepsy Program
- Boy Donates Part of His Brain to Science, Researchers Discover Major Cause of Epilepsy

