
After winning the Food Network’s Chopped Jr. reality TV cooking competition, Fuller Goldsmith, 16, was well on his way to achieving his dreams of becoming a professional chef. It was a future that was soon in jeopardy when life for the aspiring chef took an uncertain, but all too familiar turn. In late 2018, Fuller learned his cancer had returned for a fourth time.
Having undergone treatment for acute lymphoblastic leukemia (ALL) since age 3, Fuller was out of standard treatment options. Their local oncologist told the Goldsmiths about the cancer immunotherapy clinical trials at Seattle Children’s. He thought the experimental chimeric antigen receptor (CAR) T-cell immunotherapy, which engineers a patient’s own immune cells to target and eliminate cancer cells, might offer the best hope for Fuller.
“It was scary hearing the cancer had returned especially since at first, we didn’t think we had any options,” said Melissa Goldsmith, Fuller’s mother. “It was really encouraging to know there was another option out there and this wasn’t the end of the line.”
From Alabama to Seattle
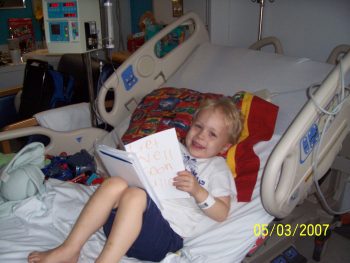
In January 2019, Fuller traveled from his home in Tuscaloosa, Alabama to Seattle Children’s where he received his reprogrammed CAR T cells as part of the Pediatric Leukemia Adoptive Therapy (PLAT-05) clinical trial.
The Goldsmiths have always recognized the value of cancer research, and so there wasn’t any hesitation when deciding to enroll Fuller on the trial.
“We want the oncologists to learn something from Fuller,” Goldsmith said. “We don’t want his suffering to be in vain, so we’ve always participated in studies since day one.”
They chose Seattle Children’s both on the recommendation of their oncologist who had trained in Seattle and because of their relationship with Dr. Todd Cooper, the director of Seattle Children’s High Risk Leukemia Program. Cooper, who previously practiced in Alabama, was Fuller’s first oncologist.
“We were heartbroken when we had to say goodbye to Dr. Cooper in Alabama,” Goldsmith said. “Part of the reason we chose to travel to Seattle for the trial is because we have close friends who live in the area; it was also comforting to know we already had a friendly face at the hospital too.”
About a month after arriving in Seattle, Fuller received his CAR T cells. Compared to his previous treatments, which most recently included a bone marrow transplant in 2011, the therapy itself and recovery seemed easy.
“With the T cells, I got to go back to our apartment every night and pretty much got to go outside whenever I wanted,” Fuller said. “It wasn’t like with the transplant where I was in the hospital for months on end.”
Like many who receive experimental CAR T-cell therapy, Fuller spiked a fever that lasted for a few days. According to Goldsmith, that was the only side effect they ever noticed.
“We spent two months in Seattle, and I think only four nights of that were in the hospital when Fuller had the fever,” she said. “It’s still hard to believe that the fourth round of treatment would be the easiest by far.”
A more robust defense
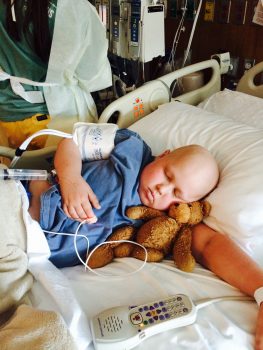
The PLAT-05 trial Fuller participated in is part of a robust pipeline of CAR T-cell immunotherapy trials at Seattle Children’s that aims to improve outcomes for childhood cancers like ALL, brain and central nervous system tumors, and solid tumors.
PLAT-05 is a phase 1 trial that tests the safety and feasibility of CAR T cells that have been reprogrammed to simultaneously target the CD19 and CD22 proteins expressed by most leukemia cells for most patients with B-cell ALL.
Early studies of CAR T-cell therapies attacking only CD19 cancer cells have shown great promise in children with relapsed and refractory ALL, but about half of patients relapse after the experimental therapy. Relapse occurs in some patients when their leukemia evolves to evade the CAR T cells by no longer expressing CD19. By targeting both CD19 and CD22 upfront, researchers hope this more robust defense will ultimately be able to cut the rate of relapse following CAR T-cell therapy by almost half.
“Standard therapies, including our more aggressive therapies like bone marrow transplant, had already failed in Fuller’s case,” said Dr. Rebecca Gardner, one of Fuller’s oncologists in Seattle Children’s Cancer and Blood Disorders Center and the principal investigator of PLAT-05. “When his cancer recurred, it was unlikely that standard therapies would successfully get him into a durable remission.”
Fuller also had leukemia cells in his spinal fluid. It’s another characteristic that made Fuller a good candidate for CAR T-cell immunotherapy.
“This likely contributed to the cancer’s recurrence as a lot of our standard therapies are not great at treating leukemia in the spinal fluid,” Dr. Gardner, said. “A relatively unique property of CAR T cells is their ability to get into non-bone marrow sites.”
Early CAR T-cell immunotherapy results in spur innovation

Today, more than a year since receiving his CAR T cells, Fuller remains in remission and his CAR T cells continue to persist.
Earlier this year, Seattle Children’s shared preliminary results from the first 28 patients enrolled in PLAT-05 as part of the 2020 American Society of Clinical Oncology Virtual Scientific Program.
Consistent with outcomes reported in the ongoing phase 1 PLAT-02 trial targeting just CD19, 85% of patients achieved complete remission. However, some patients who weren’t responding or quickly relapsed after treatment had cancers that only partially expressed CD19. With this information, researchers concluded that the CD22 component of the PLAT-05 therapy had limited activity.
“What we learned from our first patients is so important,” Dr. Gardner, said. “We had cases like Fuller, where the CAR T cells stick around for a long time, but for other patients, although the CD19 CAR T cells were likely to stay around, a lot had early loss of the CD22 CAR T cells.”
The results also indicated that the CD19 and CD22 CAR T cells could be manufactured on average in just over a week, which is important when patients have aggressive disease and need to be quickly treated. Once the CAR T cells were infused, most patients experienced only mild side effects.
“While we continue to successfully and safely get patients their CAR T cells quickly and maintain high rates of remission, we saw that we needed to make improvements to help the CD22 CAR T cells stick around for just as long as the CD19 CAR T cells,” Dr. Gardner said. “Ultimately, we hope this will lead to better outcomes for more patients.”
Based on their early learnings, the team returned to the lab to re-tool the CD22 component of the PLAT-05 experimental therapy. A new arm of PLAT-05 featuring an enhanced CD22 CAR T cell opened at Seattle Children’s last month. The trial will also reach patients at four other children’s hospitals in the U.S. and Canada as part of the CureWorks network.
The ability to quickly adapt an ongoing clinical trial is one way Seattle Children’s is accelerating the development of promising new therapies.
“We didn’t wait until the end of the trial to make this change,” Dr. Gardner said. “Instead, we took what we were observing in the clinic back to the lab, so that we could get this innovation to patients within months versus years.”
Undeterred in his dreams to become a top chef

Fuller first decided he wanted to be a chef at age 5 during one of his hospital stays for cancer treatment. He had grown tired of watching the Disney Channel and Nickelodeon in the hospital, and discovered the Food Network.
“It was calming to watch the chefs on TV cook up a storm,” Fuller said.
Soon, the budding chef was competing in cooking reality TV shows, rubbing shoulders with some of his celebrity chef idols like Guy Fieri, Curtis Stone, Richard Blais and Gail Simmons.
One of Fuller’s favorite things to do while he was in Seattle for treatment was to visit the seafood stalls at Pike Place Market.
“He was such a regular at Pike Place that they all got to know him and would call him chef,” Goldsmith said.
To keep up his craft, Fuller would carefully select a fresh piece of fish to prepare for that night’s dinner back at the apartment his family leased. Now, with cancer once again on the back burner, he is focused on his future. Fuller dreams of going to culinary school, apprenticing under a top chef and then opening not one, but two restaurants, of his own someday.
“It’s really rewarding to see kids like Fuller who are so driven toward their dreams that they don’t let cancer stand in their way,” Dr. Rebecca Gardner said. “They are undeterred in their desire to have a future.”
The Goldsmiths are thankful that Seattle Children’s helped make a future possible.
“They’re definitely the pioneers in childhood cancer and these doctors are doing amazing research,” Goldsmith said. “I’m so grateful for all that they’re doing.”
Seattle Children’s is dedicated to improving CAR T-cell immunotherapy for a variety of childhood cancers to change the odds for children with cancer. For more information about these trials, please visit our current research studies page.
If you are interested in supporting the advancement of immunotherapy and cancer research at Seattle Children’s, please visit our donation page.

