When Jacob finished his high school studies in 2015, he and his family looked for a place that would bring him a sense of community and where he could grow and learn new skills. They toured Seattle Children’s Alyssa Burnett Center (ABC). Jacob’s dad, Richard (Todd) Zenter, says the family knew immediately they were in […]
On the Pulse
In celebration of Autism Acceptance Month, Seattle Children’s Autism Center and Seattle Children’s Alyssa Burnett Adult Life Center students and families were surprised by a special visit from the Seattle Seahawks mascot, Blitz. The VIP visitors brought special sensory kits, filled with Seahawks swag, lanyards, hearing protection and more! One student family shared, “Blitz made […]
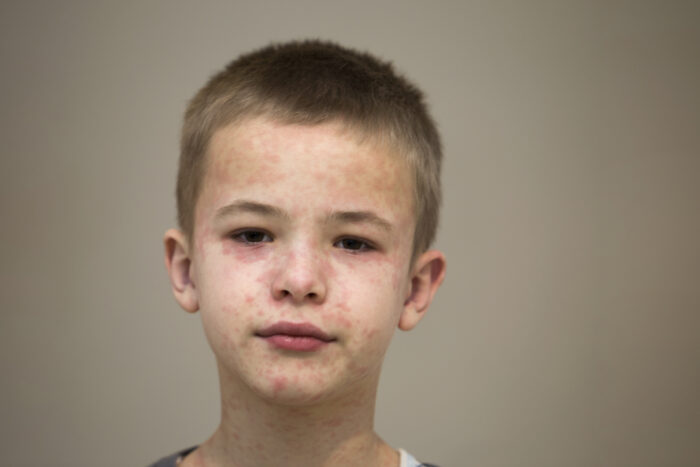
Although measles is uncommon in the United States, the number of cases across the country is increasing. In fact, the U.S. has already recorded more measles cases in 2024 than the total number of cases in all of 2023. Many other countries are seeing measles outbreaks as well, including some locations that are travel destinations. […]
Spring is a great time to check your family’s bike helmets and life jackets, to be sure they’re in good shape and still fit properly. Invest a bit of time now, before summer arrives and someone misses out on a spur-of-the-moment bike ride or boating adventure. On the Pulse shares tips on checking out the […]
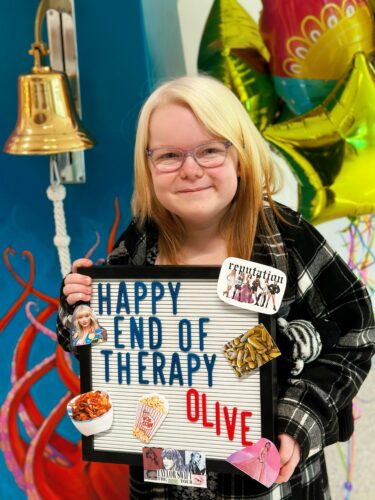
Kind and sassy Olive Ray is a typical fifth grader, with a deep love for Taylor Swift, horses, chocolate ice cream, and her little sister, January. But the 10-year-old also has some special skills, like having fun even while getting chemotherapy, raising thousands of dollars for brain tumor research and explaining how clinical trials work […]
Seattle Children’s has received FDA authorization to launch the first chimeric antigen receptor (CAR) T-cell clinical trial in the U.S. for children under 18 with the most common form of lupus.
It’s time to move clocks forward for Daylight Saving Time on Sunday, March 10. As you walk through your home to reset clocks, take another lap and test each smoke alarm using the test button. If your smoke alarms use standard batteries, be sure to change the batteries at least once each year. Choosing this […]
In February of 2024, the Seattle Children’s Heart Transplant and Cardiac Surgery teams reached a remarkable milestone moment during American Heart Month by completing 300 heart transplants since the Heart Transplant Program was established in 1994.
Skin can get dry, irritated and itchy during cold weather. Known as winter itch, this condition can be caused by cold, dry air outside plus heated air inside. Bathing habits and cleansing products can also worsen the condition.
Active kids enjoy improved mental wellness and reduce their risk of heart disease. While the days are short and the weather is often cold or dreary, kids still need to be getting physical activity each and every day. February is American Heart Month and On The Pulse asked Dr. Monique Burton, medical director of the […]