
Strokes come in many shapes and sizes. In children and adults, strokes often present sudden limb or facial numbness, confusion and dizziness.
But some strokes that cause clots to develop in the small blood vessels of the brain don’t exhibit any symptoms at all. Studies have shown that hundreds to thousands of these small, asymptomatic strokes, known as microinfarcts, likely occur over the course of decades in adult brains and may contribute to cognitive decline as we age. Even less is known about the occurrence and consequences of microinfarcts in young, developing brains.
Enter Dr. Andy Shih, principal investigator at Seattle Children’s Research Institute’s Center for Developmental Biology and Regenerative Medicine. Shih hopes to solve the mystery of microinfarcts by using advanced optical imaging — modeling them in the lab and visualizing their effects in real-time. On the Pulse sat down with Shih to learn more about his work and how he’s applying his discoveries from studying dementia in aging brains to understanding how blood vessels and clots first emerge in the brain.
Q: Your previous research studied how microinfarcts contribute to the development of dementia. How will you transfer what you learned from studying adult disease to your work at Seattle Children’s?
A: It became clear from my research in Alzheimer’s that if you don’t understand the brain’s vasculature structure at the beginning of life while studying a process that degrades with age, then you don’t have a full picture of what’s really happening. Since moving my lab to Seattle, my research looks to understand how blood vessels in the brain first develop, and how microinfarcts affect blood vessels and brain function across a lifetime.
In a previous collaboration with researchers at Emory University, we discovered spontaneously occurring microinfarcts in a mouse model of sickle cell disease – a genetic disorder that causes curvature of red blood cells that can sometimes form clots.
This is interesting because we know that about 40% of children with sickle cell disease develop small strokes visible by MRI, commonly referred to as silent cerebral infarcts. Some of these strokes are tiny enough to be categorized as microinfarcts, they’re just masquerading under a different name. Recent clinical studies suggest that microinfarcts are associated with greater cognitive decline, and so one hypothesis is that microinfarcts in sickle cell disease may contribute to cognitive impairment sometimes seen in children with the disease.
Q: How do you study microinfarcts in your lab?
A: Microinfarcts in human brains go largely undetected because they lie below the resolution limit of clinical MRI scans. Oftentimes their telltale lesions can only be seen in post-mortem brain tissue.
Because of this barrier, coupled with the infrequency of routine MRI brain scans on babies, the landscape to study the consequences of microinfarcts in developing brains is largely uncharted territory.
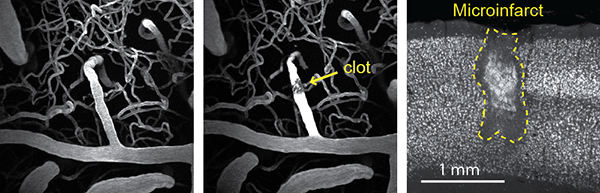
One study I am leading models microinfarcts in young mice. We use these models to visualize the vascular effects of microinfarcts in real-time using a method called photothrombosis – a minimally invasive procedure that combines photosensitizing dye and light to generate targeted clots within small blood vessels in the brain.
By using this precise approach and a multi-photon microscope, we can see what happens to the vessel and tissue surrounding it almost immediately, as well as its effects weeks or months later. We hope to learn more about what microinfarcts do to the brain; how do they impact behavior; and how they affect tissue near the injury, as well as tissue that is further away but somehow connected to that area of the brain.
Q: What are some of your early findings?
A: Preliminary study results show that although microinfarcts are small, they have broad effects. Each clot creates lasting deficits 12 times the volume of the lesion, damaging the white and gray matter in the brain and causing neuroinflammation and depression of neurovascular function.
Gray matter tissue is dense with cell bodies of neurons and vital for communication between areas of the brain. On the outskirts of gray matter are nerve fibers, or axons, that conduct electrical transmissions away from the nerve cell body. These axons are covered in myelin, known as white matter due to its lipid-rich membrane, which help increase the speed of these electrical transmissions.
We’re now focusing our efforts specifically on white matter – strategically placing microinfarcts in gray matter tissues and studying how they affect the function and structural integrity of the neighboring white matter.
I like to think of gray matter as the houses residing in the brain and white matter as the telephone wire bundles that connect them together. You only need one microinfarct nearby to jam up the entire communication between otherwise normal and viable tissues in the brain.

