As a high school freshman, Liesel Von Imhof had a dream of attending college at Harvard. She packed her schedule with challenging classes and participated in varsity sports such as cross-country running and cross-country skiing. She had occasional, debilitating headaches that sometimes caused her to miss school, but she blamed them on stress, dehydration or […]
When Cassie Fannin was 19-weeks pregnant with her first baby, she couldn’t wait for the ultrasound that would reveal her child’s gender. During the appointment, she and her husband, Michael, were delighted as they watched their beautiful baby wiggling around on the ultrasound screen. Fannin asked the technician, “Is it a boy or girl?” But […]
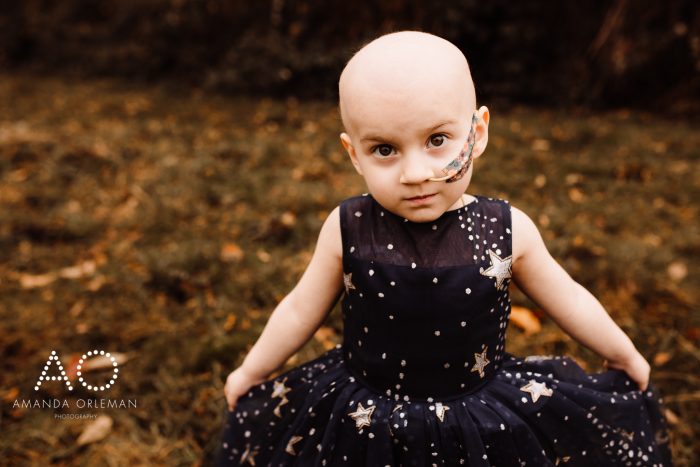
Mercy Haub has wanted to cure cancer since she was 7 years old. “The irony of it all is unbelievable,” she said. Today, at 16 years old, that mission still drives her, but now it hits closer to home, more so than she could have ever imagined. A week before the statewide lockdown went into […]
On July 19, 2017, Kelli and Dennis Williams sat in a pre-op room at Seattle Children’s with their 22-month-old son, Isaac. Kelli hugged her little boy close. He was dressed in a yellow hospital gown, happily playing with the iPad Child Life had loaned him. Kelli and Dennis did their best to appear calm in […]
There are two fateful phone calls Robin Lawrence will always remember. The first, an unexpected late-night call from her son’s pediatrician. He had just reviewed the results from his recent blood work, and something was off. The doctor instructed Robin to immediately take then 13-month-old Henry to the nearest children’s hospital to get it checked […]
Rachel Robinson and her family were on a family camping trip when she started to notice something was amiss with her son, Eli. He appeared pale, a hint of green to his complexion, and he was covered in bruises. His identical twin seemed fine, which added to Robinson’s concern. She called their pediatrician, and they […]
This year has been especially difficult for patients and families at Seattle Children’s. Spending time in the hospital is typically not a fun experience, and so for families who have to be inpatient during the holidays, the season may not feel as merry. To help spread joy and brighten up the holidays for children in […]
At 19, Faye was not planning for parenthood. She was a freshman at Northeastern University in Boston, Massachusetts. She spent her days thinking about majoring in French and dreaming of working with the United Nations Refugee Agency, not about whether she wanted to be a mother one day. But an unexpected diagnosis changed all that. […]
Tuesdays are 2-year-old Malachi Stohr’s favorite days. Every Tuesday, rain or shine, Whitney Stohr and Malachi bundle up and wave to the garbage men as they empty the garbage bins at the end of the driveway. Malachi and Whitney then take a walk around the neighborhood, following the big green truck along its route. Malachi […]
Before his first breath, Colton Iverson had already received the gift of a lifetime. Just days old, he became the youngest patient to go on a drug recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of a life-threatening genetic condition called very long-chain acyl-CoA dehydrogenase, or VLCAD, deficiency. For his […]