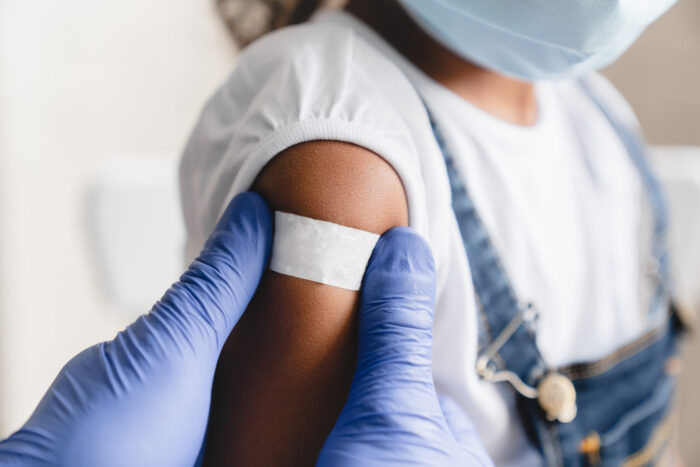
As summer starts to cool down, parents are all too familiar with the return of back to school prep and fun fall activities. While families are busy checking off school supply lists and spending more time indoors through the chillier months, it might be easy to overlook the preventive care to help keep children safe […]
This story is part two of an On the Pulse series. Read part one here. Seattle Children’s Research Institute (SCRI) is one of the nation’s leading pediatric research facilities, with talented investigators who have made stunning breakthroughs in their ongoing efforts to help every child live a full, healthy life. Working to inspire and develop […]
This story is part one of an On the Pulse series. Read more in part two. In the downtown labs of Seattle Children’s Research Institute (SCRI) this summer, 49 capable and curious college students are working side by side with scientists they might join as peers one day, demonstrating a summer job can not only […]
Founded in 2022, the Invent at Seattle Children’s Postdoctoral Scholars Program is a first-in-the nation postdoctoral training program that aspires to develop novel therapeutics at Seattle Children’s. The program is an investment in training talented early career scientists historically underrepresented in biotech in the development of therapeutics for childhood diseases. It seeks to improve the […]
Eight cases of locally acquired malaria have been confirmed in Florida and Texas this summer, marking the first time in 20 years locally transmitted cases have been seen, and decades since malaria was officially eradicated in the United States. Although about 2,000 Americans are diagnosed with malaria each year, those cases are linked with travel […]
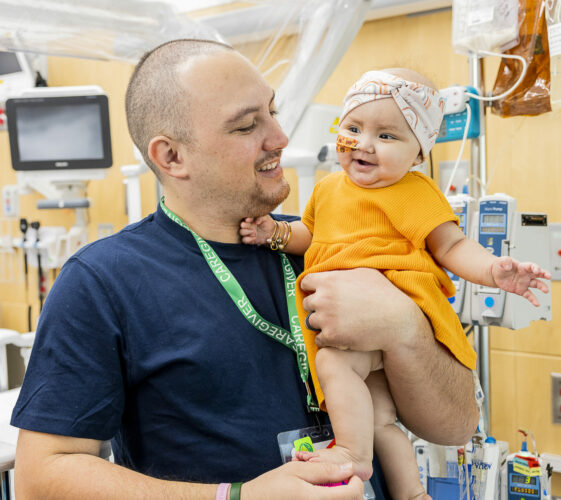
In Seattle Children’s Cancer and Blood Disorders Center (CBDC), patients are treated for some of the most complex and rare conditions seen in children, teens and young adults. Close ties and dual roles between the CBDC care team and Seattle Children’s researchers extends a unique, team approach to treating pediatric patients in their fight against […]
As one of the nation’s top four pediatric research centers, Seattle Children’s Research Institute remains dedicated to providing hope, care and cures to help every child live the healthiest and most fulfilling life possible. On the Pulse takes a look at some of the highlights from across the research institute over the past month.
The White House recently convened patients, caregivers, oncologists, researchers and administration officials for the Cancer Moonshot Brain Cancers Forum as the administration moves to advance progress for patients with glioblastoma (GBM) and diffuse intrinsic pontine glioma (DIPG). Among the attendees invited was Dr. Nicholas Vitanza, an attending physician in Seattle Children’s Cancer and Blood Disorders […]
It’s been 12 years, but Brandy Epling still chokes up at the traumatic memory of her firstborn’s birth. It was a difficult pregnancy, with preterm labor forcing a 33-day stay at a southwest Washington hospital for the mom-to-be, followed by months of bedrest. Ultrasounds revealed the baby’s brain was a bit bigger on the left […]
Seattle Children’s Therapeutics has launched BrainChild-04, a first-in-human phase 1 clinical trial that will be our first chimeric antigen receptor (CAR) T-cell clinical trial that targets four antigens at the same time, by delivering CAR T cells directly to the brain. The trial is for children, teens, and young adults with diffuse intrinsic pontine glioma […]