Seattle Children’s has received FDA authorization to launch the first chimeric antigen receptor (CAR) T-cell clinical trial in the U.S. for children under 18 with the most common form of lupus.
In August 2020, just after her third birthday, Aisley began to experience concerning symptoms: headaches in the back of her head, a lack of appetite and vomiting. After a visit to her pediatrician, Aisley was sent to Seattle Children’s for an urgent neurology visit, where she passed the exam with “flying colors,” showing no signs of trouble. Still, Dr. […]
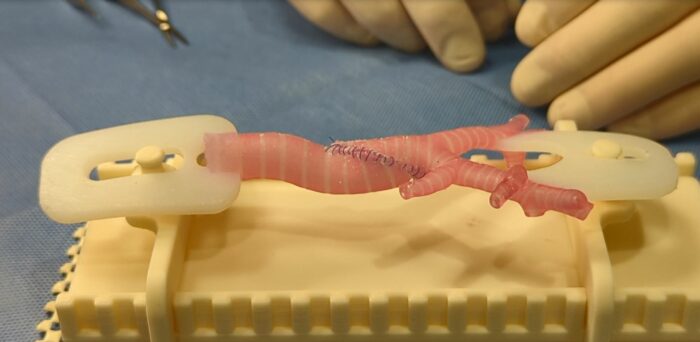
Seattle Children’s Doctors Use 3D Printing to Help Train International Care Teams on Complex Surgery
Healthcare organizations are increasingly looking to incorporate the use of 3D-printed models to help improve outcomes for pediatric patients in need of very complex surgeries. Doctors at Seattle Children’s are now utilizing 3-D printed models created in the hospital’s Innovation Lab to train international care teams in a highly specialized procedure, known as a slide […]
In a major moment for combatting respiratory syncytial virus (RSV), on Sept. 22 the Centers for Disease Control (CDC) recommended an RSV vaccine for pregnant persons that researchers have determined is safe and effective in preventing RSV disease in infants through immunization during pregnancy. The new Pfizer RSV vaccine joins the recently approved monoclonal antibody, […]
Seattle Children’s has appointed Dr. Vittorio Gallo as senior vice president and chief scientific officer. As senior vice president and chief scientific officer, Dr. Gallo will serve as the principal scientific executive of the health system and partner with Chief Research Operations Officer Dr. Eric Tham and Chief Academic Officer Dr. Leslie Walker-Harding to provide […]
Founded in 2022, the Invent at Seattle Children’s Postdoctoral Scholars Program is a first-in-the nation postdoctoral training program that aspires to develop novel therapeutics at Seattle Children’s. The program is an investment in training talented early career scientists historically underrepresented in biotech in the development of therapeutics for childhood diseases. It seeks to improve the […]
After a family trip to Finland last summer, Jasmine’s family became increasingly concerned about some unusual symptoms she was experiencing. What started as occasional numbness in her lip or left side of her face last spring had progressed to twitching, which the family later learned were increasingly strong seizures. Her family had been proactive in […]
As one of the nation’s top four pediatric research centers, Seattle Children’s Research Institute remains dedicated to providing hope, care and cures to help every child live the healthiest and most fulfilling life possible. On the Pulse takes a look at some of the highlights from across the research institute over the past month.
Seattle Children’s Therapeutics has launched BrainChild-04, a first-in-human phase 1 clinical trial that will be our first chimeric antigen receptor (CAR) T-cell clinical trial that targets four antigens at the same time, by delivering CAR T cells directly to the brain. The trial is for children, teens, and young adults with diffuse intrinsic pontine glioma […]
When Jiana was born in August 2021, she appeared to be a typical, healthy newborn baby. “I still remember her pediatrician called her a textbook baby,” recalled Latika, Jiana’s mom. Unfortunately, that normalcy was short lived. On her first day home from the hospital, Jiana’s parents noticed their daughter was twitching. “We were first-time parents […]