Kind and sassy Olive Ray is a typical fifth grader, with a deep love for Taylor Swift, horses, chocolate ice cream, and her little sister, January. But the 10-year-old also has some special skills, like having fun even while getting chemotherapy, raising thousands of dollars for brain tumor research and explaining how clinical trials work […]
Seattle Children’s has received FDA authorization to launch the first chimeric antigen receptor (CAR) T-cell clinical trial in the U.S. for children under 18 with the most common form of lupus.
Sleep is an important part of physical and mental health, especially in school-age children and adolescents. A well-rounded sleep schedule supports healthy brain function, maintains physical and emotional balance and reduces the risk of long-term health problems. Unfortunately, about 30% of parents say their kids are not getting enough shut-eye. While sleep challenges vary, research […]
This holiday season, parents, caregivers, family and friends may be on the lookout for special gift ideas for the children in their lives. With so many choices available in person and online, it can feel overwhelming to find something that is both fun and safe. Depending on a child’s age, there are certain toys with […]
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common neurodevelopmental disorders that affect children. While patients may first be diagnosed in childhood, ADHD often persists and can be diagnosed at any age. Management of the condition varies as symptoms manifest differently through various milestones in a person’s life. Common ADHD symptoms include trouble […]
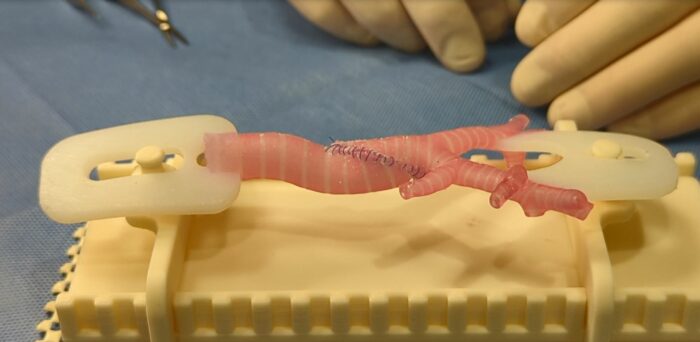
Seattle Children’s Doctors Use 3D Printing to Help Train International Care Teams on Complex Surgery
Healthcare organizations are increasingly looking to incorporate the use of 3D-printed models to help improve outcomes for pediatric patients in need of very complex surgeries. Doctors at Seattle Children’s are now utilizing 3-D printed models created in the hospital’s Innovation Lab to train international care teams in a highly specialized procedure, known as a slide […]
Dr. Molly Taylor recently found a long-forgotten journal from her college years. In it, she asked her young adult self: “Do I want to be a pediatric oncologist?” It turns out she did, and she went to medical school after studying psychology and microbiology as an undergrad. Bringing together of all those disciplines, today she’s […]
In a major moment for combatting respiratory syncytial virus (RSV), on Sept. 22 the Centers for Disease Control (CDC) recommended an RSV vaccine for pregnant persons that researchers have determined is safe and effective in preventing RSV disease in infants through immunization during pregnancy. The new Pfizer RSV vaccine joins the recently approved monoclonal antibody, […]
Seattle Children’s has appointed Dr. Vittorio Gallo as senior vice president and chief scientific officer. As senior vice president and chief scientific officer, Dr. Gallo will serve as the principal scientific executive of the health system and partner with Chief Research Operations Officer Dr. Eric Tham and Chief Academic Officer Dr. Leslie Walker-Harding to provide […]
It’s estimated nearly 86,000 adolescents and young adults (AYAs) will be diagnosed with cancer this year; in fact, one-third of patients treated for cancer at Seattle Children’s are AYAs. These 15- to 39-year-olds face the unique challenges of their life stage — finishing high school or college, starting a career, establishing independence, finding a romantic […]